MAPGuideⓇ
Equitable Access Toolkit
Approaches to Intellectual Property Rights Management
in Funding Agreements
The term intellectual property (IP) can be used to describe proprietary information such as patents, trademarks, copyrights, trade secret rights, data, research information, and know-how that is owned by an organization or individual. IP management provisions in product R&D funding agreements need to address:
1. Ownership of the pre-existing and newly developed IP needed to support the project;
2. If and how IP will be protected; and
3. If and how IP will be shared through licensing and technology transfer agreements.
As outlined in the flowchart below, there are different potential approaches to each of these elements.
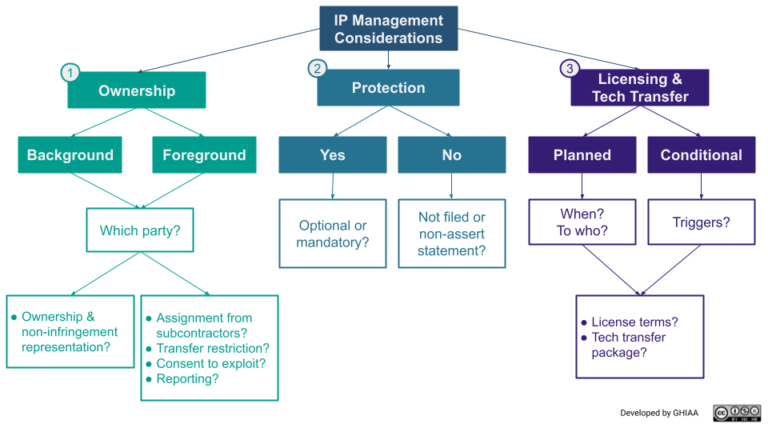
1. IP Ownership
Partnering agreement provisions related to ownership of IP need to consider the background and foreground IP relevant to a project.
a. Background IP: the most common approach found in funding agreement provisions is that the party that owns the background IP prior to the effective date of the agreement retains ownership. In the case of an agreement between a funder and developer, this party is usually the developer. It is important to ensure that the developer owns, or has rights to use, all of the background IP necessary to conduct the project activities (“freedom to operate”). Some partnering agreements require specific representations from the developer to this effect.
Examples from the MAPGuide
[Developer] shall retain ownership of its intellectual property existing as of the Effective Date, or developed or acquired independently of the Project during the term of this Agreement (“[Developer] Background IP”) and licenses to third party intellectual property secured prior to the Effective Date [***] (“Third Party Background IP” which, along with [Developer] Background IP, shall be referred to as “Background IP”), and nothing in this Agreement shall be deemed to assign any ownership in, or grant a license to, [Funder] with respect to such Background IP; except for the limited license rights otherwise expressly provided herein for the Public Health License.
Source: taken from a development funding agreement between CEPI (Funder) and Novavax (Developer) for a COVID-19 vaccine. Read in context.
To [Developer]’s knowledge, [Developer] owns or has and will own or have a valid license to all Essential Background Technology necessary for the development of the [product] in accordance with the terms of this Agreement and the Grant Agreement. To [Developer]’s knowledge as of the date hereof, the manufacture or sale of the [product] by [Developer] in accordance with this Agreement does not infringe any intellectual property rights of a third party to which [Developer] does not hold a valid license. Except to the extent expressly provided in this Agreement and the Grant Agreement, nothing in this Agreement shall be construed to confer any ownership interest, license or other rights upon the [Funder] or any of its Affiliates or any [Funder]-supported entity by implication, estoppel or otherwise as to any technology, intellectual property rights, products or materials of [Developer] or any other entity.
Source: taken from a global access and price commitments agreement between the Bill & Melinda Gates Foundation (Funder) and Icosavax (Developer) under which Icosavax makes commitments to use its expertise and experience in the development of vaccines in projects funded by the Foundation related to providing public sector purchasers with accessible vaccines for eligible countries. Read in context.
b. Foreground IP: as with background IP, ownership of foreground IP (also referred to as project IP, project results or project technology) usually remains with the developer (the IP inventor). However, some agreements include an option for the developer to assign ownership of the IP to the funder – this approach can be found in some US government funding agreements. If a funding agreement is for a collaboration, rather than a single developer, the IP ownership provision will need to consider whether ownership is held jointly between the collaborating parties. This may depend on the jurisdiction under which the IP is generated, although collaborations across jurisdictions may cause complications due to the different legal meanings that may be given to joint ownership.
Other considerations with foreground IP may include:
- Requiring that IP that is created by or with subcontractors/sub-awardees under the partnering agreement is assigned to the developer. This assignment can be important in the event that a funder wants to enforce their rights to a license;
- A notification or consent requirement prior to exploiting the foreground IP, particularly for for-profit purposes. Such consent requirements may be related to ensuring that the terms of any IP licenses contain provisions ensuring flow through of funding terms, or to ensure that the funder receives a share of commercial benefits proportionate to their funding of the product;
- Ensuring that the obligations of the agreement are transferred to a new owner if there is a transfer of ownership of project-related IP, as discussed here; and
- Notification requirements for the generation of new project-related IP, as discussed here.
Examples from the MAPGuide
Unless provided otherwise in a specific Project Agreement (as defined below), the Parties agree that any results, information, invention, patent right and other intellectual property right and any know how generated by or on behalf of the [Developer] with respect to the Platform Technology and/or the Funded Developments shall be owned by the [Developer] and the [Developer] shall be responsible, in its sole discretion, to file, prosecute, maintain and defend such intellectual property rights.
Source: taken from a development funding agreement between CEPI (Funder) and Novavax (Developer) for a COVID-19 vaccine. Read in context.
[Developer]’s obligations.
(1) The [Developer] shall disclose in writing each subject invention to the Contracting Officer within 2 months after the inventor discloses it in writing to [Developer] personnel responsible for patent matters. The disclosure shall identify the inventor(s) and this contract under which the subject invention was made. It shall be sufficiently complete in technical detail to convey a clear understanding of the subject invention. The disclosure shall also identify any publication, on sale (i.e., sale or offer for sale), or public use of the subject invention, or whether a manuscript describing the subject invention has been submitted for publication and, if so, whether it has been accepted for publication. In addition, after disclosure to the agency, the [Developer] shall promptly notify the Contracting Officer of the acceptance of any manuscript describing the subject invention for publication and any on sale or public use.
(2) The [Developer] shall elect in writing whether or not to retain ownership of any subject invention by notifying the Contracting Officer within 2 years of disclosure to the agency. However, in any case where publication, on sale, or public use has initiated the 1-year statutory period during which valid patent protection can be obtained in the United States, the period for election of title may be shortened by the agency to a date that is no more than 60 days prior to the end of the statutory period.
[…]
[Funder]’s Rights.
(1) Ownership. The [Developer] shall assign to the agency, on written request, title to any subject invention-
(i) If the [Developer] fails to disclose or elect ownership to the subject invention within the times specified [above], or elects not to retain ownership; provided, that the agency may request title only within 60 days after learning of the [Developer]’s failure to disclose or elect within the specified times.
[…]
Source: taken from an agreement between the US Government (Funder) and Novavax (Developer) for the rapid research, development and large-scale manufacturing of a COVID-19 vaccine. Read in context.
If Project IP is conceived or reduced to practice (in the case of patentable Project IP), authored (in the case of Project IP subject to copyright) or contributed to (in the case of all other forms of Project IP) during activities carried out under the Research Plan solely by or on behalf of a single Party, then such Party solely owns such Project IP. If Project IP is conceived or reduced to practice, authored or contributed to, as the case may be during activities carried out jointly by or on behalf of two (2) or more Parties under the Research Plan, then such Parties jointly and equally own such Project IP (“Joint Project IP”), unless otherwise agreed between them. Inventorship of Project IP is determined in accordance with applicable national law where the invention is made.
Source: taken from an anonymised agreement for a collaboration between an academic institution and a company, supported by a funder. Read in context.
Two or more [Developers] own results jointly if:
(a) they have jointly generated them and
(b) it is not possible to (i) establish the respective contribution of each [Developer], or (ii) separate them for the purpose of applying for, obtaining or maintaining their protection.
The joint owners must agree (in writing) on the allocation and terms of exercise of their joint ownership, to ensure compliance with their obligations under this Agreement.
Unless otherwise agreed in the joint ownership agreement, each joint owner may grant non-exclusive licenses to third parties to exploit jointly-owned results (without any right to sub-license), if the other joint owners are given:
(a) at least 45 days advance notice and
(b) fair and reasonable compensation.
Once the results have been generated, joint owners may agree (in writing) to apply another regime than joint ownership (such as, for instance, transfer to a single owner (see Article [x]) with access rights for the others).
Source: taken from a model grant agreement for use between the Innovative Medicines Initiative Joint Undertaking (IMI – now transitioned to the Innovative Healthcare Initiative) (Funder) and multiple beneficiaries (Developers). Read in context.
The [Developer] shall use its best commercial efforts to include provisions in its contracts with its subcontractors performing service(s) requiring the subcontractors to assign to the [Developer] all Project IP Rights.
Source: taken from the template CARB-X (Funder) cost-reimbursement agreement with subrecipients (Developer) for the early stage development of antibiotics, vaccines, and rapid diagnostics against bacterial threats. Read in context.
Except as expressly provided below and to the extent feasible and legally possible, all Project Technology shall be either the property of the Partner or be licensed from Third Parties, and any patents in respect of Project Technology shall be applied for in the name of the Partner. The Partner shall procure that:
- any Affiliate, Third Party collaborator, Third Party funder, co-owner or Sub-Contractor of the Partner shall assign all its right, title and interest in Project Technology promptly to the Partner to the extent Controlled and shall retain rights in the same to the extent stipulated under the agreement between Partner and Sub-Contractor
- it shall have in place contracts with those working on or funding all Work Packages of the Project to ensure that the Project Technology shall vest in the Partner and not with any members of staff individually. Where by local applicable law such rights do vest in individual members of staff, the Partner shall ensure that it has all rights to take assignment of all right title and interest in the same and the Partner shall bear the costs of any necessary contribution to such individual or other costs of assignment; and
- where a Partner has appointed NIH or another government entity or university as a Third Party Sub-Contractor and such government entity is required by law or otherwise to retain ownership of [Developer] Results they have generated in the conduct of activities described in a Work Package Statement (“Government Results”), the Partner shall ensure that the government entity provides Partner with sufficient rights and license (including via option to license where the government entity is unable to provide licenses in advance of generation) to any such Government Results in order to enable the Partner or [Funder] to further Develop the Platform and Develop and Manufacture Project Vaccines and Manufacture Products in accordance with the terms and conditions of this Agreement.
Source: taken from a development funding agreement between CEPI (funder) and CureVac (developer) for the development of CureVac’s mRNA platform for the rapid manufacturing of vaccines against infectious diseases. Read in context.
Prior to any member of the [Developer] Group (whether itself or through any other member of the [Developer] Group, or by granting a license or in collaboration with any Third Party) (the “Exploiting Party”), commencing the Development and/or Exploitation Of any Programme Intellectual Property and/or Products both inside and outside the Field, [Developer] or the relevant the member of the [Developer] Group shall obtain the prior written consent of the [Funder] to such Development and Exploitation by sending written notice to the [Funder] and the following information:
- reasonable details of the relevant Programme Intellectual Property, the Products and the activity proposed;
- details of whether the proposed Exploitation will be on a For-Profit and/or Not-For-Profit Basis; and
- if applicable, amounts of any milestones payments and royalties that would be payable to the [Funder] pursuant to Schedule [x] [revenue sharing terms] and any other applicable terms.
Where Exploitation is to be on a For-Profit Basis, the grant of the [Funder]’s consent pursuant to Clause [x] shall be conditional on the payments to the [Funder] of amounts calculated pursuant to Schedule [x], and the [Funder] and [Developer] agreeing an appropriate share of any revenue payable to the [Funder] pursuant to Schedule [x].
Related Definitions: “Exploit” means exploitation activities after completion of the Programme including obtaining Marketing Approval and the commercialisation, licensing, marketing, distribution and sales of Programme Intellectual Property and any Products utilising the Programme Intellectual Property including on a Not-for-Profit Basis and “Exploited” and “Exploitation” shall be construed accordingly.
Source: Taken from a research funding agreement between the Wellcome Trust (Funder) and PTC Therapeutics (Developer) under the Trust’s Seeding Drug Discovery Strategic Award Programme for the discovery and development of a novel glioblastoma treatment. Read in context.
In order to ensure that any proposed exploitation is in accordance with the FUNDER’s Mission, the Partner shall obtain FUNDER’s prior written consent before exploiting any of the Foreground Intellectual Property or any Product. FUNDER shall only withhold its consent to exploitation:
- where the proposed exploitation in the Field in the Affected Territory is inconsistent with the FUNDER Mission, the FUNDER Policies or the provisions of this Clause;
- FUNDER has material concerns about the capability, solvency or reputation of any third party who is proposed to be involved in the exploitation; or
- the Partner plans to transfer the Foreground Intellectual Property to a third party but does not also intend to transfer to the third party the Partner’s obligations to FUNDER under this Agreement in such a way that FUNDER could enforce such obligations directly against such third party.
Exploitation outside the Field or outside the Affected Territory. Where any proposed exploitation by the Partner is either:
- in the Field, but with Development and Marketing Activities directed outside the Affected Territory; or
- outside the Field and for Development and Marketing Activities directed outside the Affected Territory;
FUNDER’s consent shall be conditional on the following: (i) the Partner shall be the sponsor of any clinical trial of a pharmaceutical composition which infringes the Foreground Intellectual Property (a “Similar Product”) unless FUNDER otherwise agrees in writing; (ii) the Partner shall consult with and agree the protocol for such clinical trial with FUNDER in advance and shall not proceed with any such clinical trial without FUNDER’s approval, such approval not to be unreasonably withheld, conditioned or delayed; (iii) the Partner shall communicate to FUNDER in writing any data relating to a Similar Product of which it becomes aware which discloses a serious adverse event, promptly (and in any event within forty-eight (48) hours) and where that serious adverse event is a suspected, unexpected, serious adverse reaction or death or raised any other material safety signal, immediately; (iv) any relevant event under any pharmacovigilance activities and (v) shall grant FUNDER a right of reference to the regulatory materials relating to any and all Similar Products.
Source: taken from an anonymised product development funding agreement. Read in context.
2. IP Protection
The protection of intellectual property generally refers to the filing and enforcement of patents to protect existing and newly created inventions related to a project. Taking steps to protect IP can be beneficial because it can help early stage research organizations secure development partners. Developers may also protect IP in order to ensure that their competitors do not act first to obtain patent rights which could then act as a barrier to meeting equitable access objectives. IP is also a valuable asset that can have an impact on commercial product developers’ ability to raise future funding. For these reasons, partnering agreements often require that patents are filed or allow developers to do so at their discretion.
In some cases, however, the equitable access objectives of a partnering agreement may be better served if project-related IP is not protected. This could facilitate manufacturing of a product by a range of organizations, therefore increasing availability and stimulating price competition. Where this approach is followed by a partnership, agreements can either require that no patents are filed for project-related IP in certain territories, or they can include a “non-assert” or “non-suit” statement which confirms that the developer will not enforce its patents in certain territories.
Examples from the MAPGuide
[Developer] shall not abandon any patent application, patent or other right included within the scope of the Project Intellectual Property or the Background Intellectual Property, the effect of which would be to limit or prevent [Funder] from practicing the intellectual property rights licensed to [Funder] under this Agreement, without disclosure to [Funder] and arrangement by mutual agreement with [Funder] for the protection of [Funder] license rights.
Source: taken from a development funding & collaboration agreement between PATH (Funder) and Aridis (Developer) for the formulation development of a rotavirus vaccine. Read in context.
Each [Developer] must examine the possibility of protecting its results and must adequately protect them — for an appropriate period and with appropriate territorial coverage — if: (a) the results can reasonably be expected to be commercially or industrially exploited and (b) protecting them is possible, reasonable and justified (given the circumstances).
[Funder] ownership to protect the results. When deciding on protection, the [Developer] must consider its own legitimate interests and the legitimate interests (especially commercial) of the other [Developers].
If a [Developer] intends not to protect its results, to stop protecting them or not seek an extension of protection, the [Funder] may — under certain conditions (see Article x) — assume ownership to ensure their (continued) protection.
Source: taken from a model grant agreement for use between the Innovative Medicines Initiative Joint Undertaking (IMI – now transitioned to the Innovative Healthcare Initiative) (Funder) and multiple beneficiaries (Developers). Read in context.
If electing title, [Developer] will file its initial patent application on a Subject Invention to which it elects to retain title within one year after election of title, or, if earlier, prior to the end of the statutory period wherein valid patent protection can be obtained in the United States after a publication, or sale, or public use.
Foreign Patent Filing Requirements: [Developer] may elect to file corresponding patent applications in additional countries outside the U.S. (including but not limited to the European Patent Office and the Patent Cooperation Treaty) at its discretion. To enable the Government to protect patent rights in Subject Inventions in all potential countries, [Developer] shall inform the Government of which countries [Developer] shall NOT file patents applications in for all elected Subject Inventions at least 3 months prior to the filing deadline therein (e.g., in PCT national stage applications, at least 3 months prior to the PCT national stage filing deadline). [Developer] understands that, in case the Government decides to file in a particular country following a notice provided under the preceding sentence, all ownership and interest in Subject Inventions in said countries (where [Developer] shall not file patent applications in) are thereby automatically conveyed to the Government, and [Developer] agrees to timely execute any documentation required to effectuate this conveyance, so as to enable the Government to perfect patent filing in said countries, always subject to the license described in Article IX, Section 5(a).
If [Developer] does not elect title via the process described above, or determines that neither it nor its designee intends to file any patent applications on a Subject Invention, [Developer] shall notify the Government, in writing, within 6 months of disclosure of such Subject Invention to the Government. [Developer] agrees, in such case, to permit the Government to elect title to said Subject Invention, and thereby have full rights and ownership therein, at least 3 months PRIOR to the end of the one (1)-year statutory period wherein valid patent protection can still be obtained in the United States after any publication, sale, or public use.
Source: Taken from an agreement between the US Government (Funder) and Janssen Pharmaceuticals (Developer) for the rapid research, development and large-scale manufacturing of a COVID-19 vaccine. Read in context.
Background Technology. Partner shall have the right but not the obligation to prosecute, maintain and defend the patent rights which are part of the Background Technology.
Project Technology. The Partner has the rights but no obligation to take responsibility for seeking and maintaining protection for Project Technology at its sole cost, including the filing, prosecution, maintenance, extension and defense of any patent applications or patents in respect of Project Technology.
Source: taken from a development funding agreement between CEPI (Funder) and CureVac (Developer) for the development of CureVac’s mRNA platform for the rapid manufacturing of vaccines against infectious diseases. Read in context.
After the EFFECTIVE DATE, LICENSEE shall be responsible for all future costs of filing, prosecution and maintenance of all foreign patent applications, and patents contained in the LICENSED PATENTS in the countries outside the United States in the LICENSED TERRITORY selected by [LICENSOR] and agreed to by LICENSEE or requested by LICENSEE. All such applications or patents shall remain the property of [LICENSOR]. If [LICENSOR] enters into any license agreements with third parties to any of the LICENSED PATENTS, then the patent expenses in this Article 10 shall be shared equally amongst LICENSEE and such third party licensees according to the formula I/N, where N = the total number of licensees (including LICENSEE) to the applicable LICENSED PATENTS. LICENSEE acknowledges that [LICENSOR] shall not file any such applications in low or lower-middle income countries, as designated by the World Bank (www.worldbank.org).
Source: taken from a license agreement between Yale University (Licensor) and BIND Biosciences Inc (Licensee) in relation to certain Yale intellectual property rights relating to targeted and high density drug-loaded polymeric materials to develop and commercialize licensed products and to practice licensed methods for the treatment of cancer. Read in context.
University and Licensee on behalf of themselves and any successors-in-interest to the Licensed Products and Licensed Processes covenant that they will not, before or after the Effective Date of this Agreement, assert any claim of patent infringement (including direct infringement, contributory infringement, and induced infringement) under any of the patents in the Patent List, any Licensed Processes or any Orange Book Patent Right for manufacture, use, sale, offer for sale or importation of Licensed Products against any third party engaged in the manufacture, use, sale offer for sale, or importation of Licensed Products in or for Non-Suit Countries for sale to Public Sector entities. The above notwithstanding, this non-suit provision will only apply to products which when offered for sale to End Users are in a Trade Dress that is different from Licensee’s Trade Dress in every respect.
Related Definitions:
- “Non-Suit Countries” means all countries other than Market Countries.
- “Market Countries” means: (a) All current and future Organization for Economic Cooperation and Development (OECD) countries, presently consisting of Australia, Austria, Belgium, Canada, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Republic of Korea, Japan, Luxembourg, Mexico, the Netherlands, New Zealand, Norway, Poland, Portugal, Spain, Sweden, Switzerland, Turkey, the UK, and the United States; and (b) All current and future members of the European Union not otherwise members of the OECD; and (c) People’s Republic of China, India, Malaysia, Russian Federation, Singapore, South Korea and Taiwan].
Source: taken from Boston University’s template for exclusive license agreements with small entities. Read in context.
3. Licensing & Technology Transfer
There are different approaches available for the inclusion of provisions in funding agreements that require the developer to grant licenses to its background and/or foreground IP to either a funder or a third party. Agreement provisions related to license grants need to address a number of questions related to the scope of the license (discussed in more detail here), but some key points to be considered for product development funding agreements are:
-
- Should the developer be required to identify and grant a license to a secondary manufacturer as part of the scope of the funded project?
- Should there be a contingent license grant that is triggered if the developer does not meet it’s obligations?
- What are the definitions of background and foreground IP for which licenses are granted, what additional technology transfer obligations are required to ensure that sufficient data and knowledge, beyond the IP that is protected by patents, is provided to the licensee?
a. Required identification of a secondary manufacturer: The aim of these obligations is often to ensure that there is at least one manufacturer that will be willing and able to meet the affordability and supply needs of lower-income countries on an ongoing basis. As an alternative requirement for a direct license grant to a manufacturer, funding agreements could require the negotiation of a license with a patent pool (for example, MPP) which would then be responsible for granting sublicenses to suitable manufacturers. Additional requirements for the selected manufacturer(s) to be located in an LMIC can also support the broader development of local manufacturing capacity, enabling the generation of longer-term impact from a funder’s investment.
Examples from the MAPGuide
To facilitate achievement of the [equitable access] conditions set out in Clauses [x], [Developer] has agreed to transfer its technology to an LMIC manufacturer as outlined in the [product development plan]. Without limiting [Developer]’s obligations under the IPDP, [Developer] will, within [***] of the signature date of this Agreement, or within such other time period as may be set out in the IPDP if the IPDP is amended in accordance with Clause [x], sign a Sub-Award agreement with an LMIC manufacturer, which Sub-Awardee agreement shall meet the requirements of Clause [x] and shall obligate such LMIC manufacturer to manufacture the Product for regular supply in all Non-Traveler’s Market Countries that have a demand for Product and to supply the Product to Non-Traveler’s Market Countries under the conditions of Clause [x]. Prior to signing such Sub-[Developer] agreement with an LMIC manufacturer and prior to completion of technology transfer to enable such LMIC manufacturer to manufacture and supply the Product to Non-Traveler’s Market Countries, [Developer] shall fulfill manufacturing and supply obligations for Non-Traveler’s Market Countries as set out in the IPDP.
Source: taken from a development funding agreement between CEPI (Funder) and Valneva (Developer) for manufacturing and late-stage clinical development of a Chikungunya vaccine. Read in context. The required agreement for an LMIC manufacturer was subsequently signed between Valneva and Instituto Butantan.
The [Developer] will work with the Foundation to develop (by the time of completion of Phase II clinical trials) and execute a manufacturing and supply plan that will enable to be met the reasonably expected demand in Developing Countries for any Products. […] The manufacturing and supply plan could involve the use of manufacturing partners and support from donors, and the specific level and allocation of funding responsibilities in such plan will be decided as mutually agreed in good faith in writing by the parties based on a fair allocation of the expected benefits between Developing Countries and developed countries. […]
Source: taken from a strategic relationship agreement between the Bill & Melinda Gates Foundation (Funder) and Arsanis (Developer) in connection with an $8 million investment by Gates to support a Staphylococcus aureus antibody development program. Read in context.
b. Contingent license grants: The purpose of these provisions is to support the achievement of the equitable access objectives of a funding agreement if a developer does not or cannot meet its obligations. The license grants may come in the form of ‘Access Licenses’ that can be exercised by the funder, or more general requirements to grant a license to a third party if the developer cannot meet its price or volume commitments.
Examples from the MAPGuide
If the [Funder] reasonably determines that a third-party manufacturer is needed to achieve [Developer’s] price and volume commitments, [Developer] must license and transfer the IP needed for production to the third party at the [Funder’s] expense. The obligation is limited to transfers that allow production for Developing Countries.
Source: taken from a strategic relationship agreement between the Bill & Melinda Gates Foundation (Funder) and Arsanis (Developer) in connection with an $8 million investment by the Foundation to support a Staphylococcus aureus antibody development program. Read in context.
Subject to the undertakings to be defined in the Additional Work Packages and – upon Partner’s request, subject to a separate confidentiality agreement to be concluded between the Partner and the Trusted Manufacturer – the Partner will support [Funder] in appointing one or more Trusted Manufacturers that are technically and operationally capable of and willing to rapidly Manufacture Product for use in the Field in the Affected Territory on an ongoing basis both during and after completion of the Project, in accordance with [Funder]’s requirements, as set forth herein.
Subject to the undertakings in the Additional Work Packages the Partner shall:
(i) grant appointed Trusted Manufacturers all necessary rights to use (on a non-exclusive, royalty-free and license-fee free basis) the Background Technology and Project Technology to further Develop the Platform, and to Manufacture Products for use in the Field in the Affected Territory in accordance with the [Funder] Production Timescale and in the quantities reasonably likely to be necessary in the event of an Outbreak or risk of Outbreak in the Field and at a cost of goods in line with the methodology to determine pricing obligations set out in the [Funder] Equitable Access Policy;
Related Definitions:
- “Additional Work Package” means a Work Package of work under the Project to be agreed between the Parties from time to time in addition to those agreed upon on the Effective Date, and funded by [Funder] which may be related to Products.
- “Trusted Manufacturer” means a Third Party nominated by the Partner and appointed by [Funder], or nominated by [Funder] and appointed by the Partner if so agreed in Additional Work Package Statements.
Source: taken from a development funding agreement between CEPI (Funder) and CureVac (Developer) for the development of CureVac’s mRNA platform for the rapid manufacturing of vaccines against infectious diseases. Read in context.
c. Technology transfer obligations: these provisions need to consider the multiple types of information, data, documentation, and support that either a funder or alternative partner might need in order to successfully continue the activities of the developer. These requirements can include:
- Electronic records related to the relevant IP, technology and know-how such as master batch records, standard operating procedures (SOPs), Quality Assurance and Quality Control (QA/QC) data, a detailed bill of materials for the product;
- Access to and the rights to cross-reference regulatory documentation;
- Negotiation of access to, or assignment of, third party licenses;
- The provision of technology transfer assistance in accordance with commercially reasonable expectations and standard industry practice.
Examples from the MAPGuide
[…] [T]he Partner shall:
[…]
(ii) provide the Technology Transfer Materials to the Trusted Manufacturers and ensure that such Technology Transfer Materials are kept up to date, in particular, at each date on which the Partner requests any payment from [Funder], on the occurrence of one or more Conditions Precedent and on termination or expiration (for whatever reason) of this Agreement;
(iii) at the request of [Funder], enable Trusted Manufacturers to establish a warm base for the further Development of the Platform, and Manufacturing of Products for use in the Field in the Affected Territory;
[…]
(v) provide all necessary commercially reasonable support to the Trusted Manufacturers to facilitate the foregoing.
Related Definitions:
- “Technology Transfer Materials” means the materials required to be made available to a Trusted Manufacturer to enable such Trusted Manufacture to (i) adapt, develop and use the Platform for the Manufacture of Products for use in the Field and in the Affected Territories (ii) develop, formulate, recreate and show equivalence (where relevant) to Products developed by Partner under an Additional Work Package. For the avoidance of doubt, Technology Transfer Materials do not include RNA Optimizer Toolkit technology.
- “Trusted Manufacturer” means a Third Party nominated by the Partner and appointed by [Funder], or nominated by [Funder] and appointed by the Partner if so agreed in Additional Work Package Statements.
Source: taken from a development funding agreement between CEPI (Funder) and CureVac (Developer) for the development of CureVac’s mRNA platform for the rapid manufacturing of vaccines against infectious diseases. Read in context.
(i) In connection with any Global Access License hereunder, such Global Access License shall be subject to the execution of the following reasonably acceptable written agreements between the [Developer] and the recipient of the technology transfer (which recipient may be a [Funder] sub-licensee or entities selected by the [Funder]): quality agreement, safety data exchange agreement, and other customary agreements related to technology transfer of the Product; provided always that such entity shall not be required to pay any royalties, milestones or fees associated with such agreements. [Developer] will cooperate with the [Funder] in good faith to make available to the [Funder] (or the entities of the [Funder]’s choosing) (including providing electronic copies), all necessary intellectual property, technology, know-how and other information relating to the Product (including but not limited to master batch records, SOPs, QA/QC information, detailed bill of materials for the Product and other manufacturing documentation) for the purpose of permitting the [Funder] (or its selected entities) to utilize its Global Access License and to continue to research and develop and manufacture the Product, and to enable the manufacture, licensure, sale, offer-for-sale, import, export, distribution, and use of such Product intended for use in the Developing Countries.
For the purpose of facilitating Technology Transfer the [Developer] shall provide electronic copies of all such applicable records and manufacturing documentation related to the Product for Maternal Immunization and the [Funder] (or the entities of the [Funder]’s choosing) and will be permitted to inspect the same for the purpose of assuring complete and accurate technology transfer by [Developer].
(ii) [Developer] will continue to meet its Global Access Commitments towards and until completion of all intellectual property, know-how and information technology transfer associated with a Global Access License herein. [Developer] and the [Funder] will cooperate in good faith to effect an orderly and complete transition of any activities, including the research, development, manufacture, licensure, sale, offer-for-sale, distribution, import, export and use of the Product to the [Funder] or its selected entities.
(iii) [Developer] shall permit the [Funder] (or its sublicenses) the right to access and cross-reference any applicable IND, BLA, WHOPQ or other regulatory file relating to the Product and shall, upon request, provide an electronic copy of each such file.
(iv) To the extent applicable, the Parties further agree to take all reasonable and diligent steps to eliminate or reduce any third party costs or royalties (set forth in Appendix [x] or otherwise attributable to the Product) associated with such Global Access License, including negotiation of any third party royalties and to negotiate access to such third party licenses by the [Funder] (or its selected entities).
Source: Taken from a global access commitments agreement between the Bill & Melinda Gates Foundation (Funder) and Novavax (Developer) for the development of an affordably-priced RSV vaccine for use in maternal immunization to provide RSV protection in low income countries. Read in context.
In connection with the exercise of any license hereunder or under a grant agreement (as applicable), the [Developer] will take further actions, including technology transfer (subject to the transferee agreeing to appropriate confidentiality obligations), as would be commercially reasonable industry practice at the time with respect to providing a biotechnology license to a third party, to accommodate that the [Funder], the [Funder]’s sublicensees, and/or the relevant [Funder] supported Entity can effectively exercise the applicable license or sublicense and use the related technology (including the right to reference regulatory filings related to the applicable products).
Source: taken from a strategic relationship agreement between the Bill & Melinda Gates Foundation (Funder) and Arsanis (Developer) in connection with an $8 million investment by the Foundation to support a Staphylococcus aureus antibody development program. Read in context.
As described in the [product development plan], [Developer] will be transferring technology to two Sub-Awardees (IDT and an LMIC manufacturer) and the costs of such technology transfers are included in the Project Budget. [Developer] will promptly and diligently provide all necessary guidance, information, materials and assistance reasonably required to transfer [Developer]’s technology to each such Sub-[Awardee] as outlined in the [product development plan]. Pursuant to an Outbreak Notice, [Funder] may request to accelerate the timelines for transfer of [Developer]’s technology to one or both of such Sub-Awardees and/or [Funder] may request an expansion of the transfer to another Trusted Collaborator (other than such Sub-Awardees) if that would achieve the transfer more quickly. If [Funder] requests transfer of [Developer]’s technology to another Trusted Collaborator, [Developer] will promptly and diligently provide all necessary guidance, information, materials and assistance reasonably required by such Trusted Collaborator to accomplish the activities that may be requested by [Funder] under Clause [x] (“Technology Transfer”) at [Funder]’s cost. [Developer] shall carry out the Technology Transfer to such other Trusted Collaborator pursuant to the terms and conditions of a to-be-agreed-upon confidentiality agreement in accordance with this Agreement to be entered into between [Developer] and the Trusted Collaborator governing the Trusted Collaborator’s use and non-disclosure of information and materials provided in connection with the Technology Transfer, provided that [Developer] and the Trusted Collaborator shall not delay the execution of such agreement.
Source: taken from a development funding agreement between CEPI (Funder) and Valneva (Developer) for manufacturing and late-stage clinical development of a Chikungunya vaccine. Read in context.
Upon exercise of the Public Health License and written notice to [Developer], [Developer] [***] shall:
(a) provide [Funder] with an updated list of Enabling Rights and applicable Background IP, along with an invoice for any payments due under any license agreement for Third Party Background IP attributable to the grant of the Public Health License to [Funder] or a sublicensee;
(b) provide [Funder] with a good faith schedule of key technology transfer activities and estimated costs for the technology transfer in Clause [x];
(c) [***] transfer to the Trusted Collaborator and/or Trusted Manufacturer, as the case may be, and at [Funder]’s reasonable cost, all Project Results, Project Materials described in Clause [x], all guidance, information, materials and assistance reasonably required to accomplish the Project activities identified by [Funder]; and
(d) shall be deemed to have covenanted not to sue [Funder] or designee for the exercise of the Public Health License.
Related Definitions:
“Enabling Rights” means rights to Intellectual Property and Project Results that could be asserted by [Developer] to block [Funder] from exercising its rights under Clause [x] [project continuity] of this Agreement. For purposes of this Agreement, Enabling Rights also includes the contractual rights under contracts executed for the Project that control the use of such items, for example, in material transfer agreements.
Source: taken from a development funding agreement between CEPI (Funder) and Novavax (Developer) for a COVID-19 vaccine. Read in context.
If the [Funder] exercises its right to exploit any Programme Intellectual Property under Clause [x]:
- [Developer] will exclusively licence to the [Funder] or its nominee, the Programme Intellectual Property in such indications or regions as are specified in the notice served by the [Funder] exercising the option consistent with the applicable sub-clause in Clause [x]. The terms of such exclusive licence to the relevant Programme Intellectual Property shall:
(i) be free of consideration in respect of sales of Product made on a Not-for-Profit Basis, and
(ii) include a share of any revenue or other consideration received by the [Funder] under any license of relevantProduct Intellectual Property with respect to all other sales, such share to be based on the respective contributions made by [Developer] and the [Funder] in the Development and Exploitation of such Product;
- [Developer] will grant to the [Funder] or its nominee, a non-exclusive licence to relevant Background Intellectual Propertysolely as required and for the purposes of enabling the [Funder] to exercise the rights to the relevant ProgrammeIntellectual Property as described in (a) above and solely in the regions specified in the notice served by the [Funder]exercising the option. Any such licence grant shall be non-exclusive and free of charge other than for reasonable costs that are incurred in respect of necessary third-party licences; and
- provide the [Funder] with access to any associated data, Documents (including, without limitation, Documents relating to pre-clincial data and clinical trials), pre-clinical data, Materials (only to the extent actually in existence and amenable to transfer in reasonable quantities without further regulatory approval(s), and not to include commercial inventory of Product for which [Developer] retains rights to Exploit), regulatory approvals, Marketing Approvals, information as required for the [Funder] to exploit such rights.
If the [Funder] exercises its right to exploit any Programme Intellectual Property under Clause 12.1 above, [Developer] agrees that it shall pass (or will procure that relevant members of the [Developer] Group shall pass) to the [Funder] immediately any or all exploitation opportunities in the applicable region(s) that it becomes aware of from time to time in connection with the Programme Intellectual Property. [Developer] further undertakes that it shall not (and that it shall procure that no member of the [Developer] Group shall) engage in any activities (including in relation to the Background Intellectual Property) that could reasonably lead to the loss of an exploitation opportunity in the applicable region and with respect to the applicable indication(s) without the prior written consent of the [Funder].
Source: Taken from a research funding agreement between the Wellcome Trust (Funder) and PTC Therapeutics (Developer) under the Trust’s Seeding Drug Discovery Strategic Award Programme for the discovery and development of a novel glioblastoma treatment. Read in context.
Related Commentaries
While the protection of project-related IP can be important to the successful outcome of a partnering agreement, the parties should also consider approaches to the publication and sharing of project data in order to support further research and development in the field.
Planned license grants and technology transfer to third party manufacturers should be integrated into an access plan to ensure that the necessary selection, agreement and transfer processes are conducted in a timely manner.
This toolkit has been built based on the data in the MAPGuide and the GHIAA team’s experience of negotiating and implementing agreements. We intend that the toolkit will evolve and expand over time based on input from MAPGuide users and availability of new agreements showing examples of alternative approaches. We welcome ongoing constructive dialogue around these materials and encourage you to contact us or fill in our feedback survey to share your thoughts, questions and suggestions.
Authors: Bridie Telford
First publication date: November 21, 2022
