Provision Language
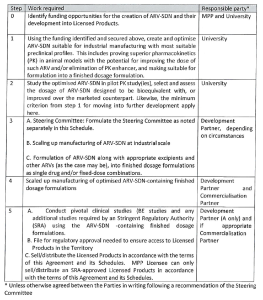
Schedule 2 – Responsibilities of the Parties
Schedule 5 – Commercialization Agreement Term Sheet
[…]
7. Waiver of data exclusivity rights: Commercialization Partner agrees, where applicable and to the extent that it is able: (a) to not seek; and (b) to waive, regulatory exclusivity in the Territory in relation to any data relating to the Licensed Products.
[…]
10. Reporting: Within 10 business days following the end of each calendar quarter, Commercialisation Partner will be required to provide MPP and the University with a quarterly written report setting forth in relation to that quarter (a) Licensed Products in its development pipeline, (b) status of development of each licensed Product in development, (c) regulatory filing plan for each licensed Product, (d) a list of countries within the Territory for which such regulatory approvals or authorizations have been filed or obtained for any Licensed Product and (e) the Licensed Products (in terms of smallest units and patient packs for each formulation) sold or supplied by the Licensee under the Commercialisation Agreement during such agreement quarter, on a country-by-country basis; f) any scientific discoveries or Know how developed related to the Licensed Technology. MPP and Licensee will agree to meet on a quarterly basis regarding such reports and also review development and filing status of Licensed Products. MPP will agree that information contained in quarterly and other such reports shall be treated as confidential; provided, however, that such information may be shared with the University; and that aggregated data may be publicly disclosed by MPP.
