Provision Language
Definitions
“CSIC New Developments” shall mean inventions and improvements conceived by CSIC or on its behalf, by its respective employees and agents after the Effective Date relating to the COVID-19 Vaccine.
“Licensed Know-how” means all know-how, information, data, including without limitation clinical data, and other technical knowledge owned and/or controlled by CSIC now or in the future, that are useful or otherwise relevant for the development and/or manufacturing of the COVID 19 vaccine, which is set out in Schedule 3 hereto as available on the Effective Date, which shall be updated and complemented from time to time by CSIC.
“Licensed Technology” means the Patent Rights, Material, and Licensed Know-how including CSIC New Developments.
“Material” means any materials useful for the development and/or manufacturing of COVID 19 Vaccine as defined above owned and/or controlled by CSIC, including the premaster virus seed for MVA-CoV2-S(3P) and unmodified derivatives.
“Biofabri Material and Know-how” means any materials and know how useful for the manufacturing and quality control of “COVID-19 Vaccines” owned and/or controlled by Biofabri:
Master virus seeds (MVS) and working virus seeds (WVS) were generated by Biofabri from CSIC Materials and adequately characterized in accordance with the regulatory guidelines.
DF1 Master Cell Seeds (MCS) and DF1 Working Cell Seeds (WCS) were generated by Biofabri in accordance with the GMP rule. MCS and WCS were characterised by Biofabri following the European Pharmacopoeia (EP) 5.2.3.
“Patent Rights” means any right recognised by the applicable patent legislation or regulation and generated by claiming the priority of the Patents and Patent Applications, including the patents and patent applications set out in Schedule 2 as may be amended from time to time, such as the rights generated by:
a) any patent application, any continuation-in-part, division, extension for any such application, and any patent issuing on such application;
b) inventor certificates, utility models and petty patents.
2. SCOPE OF THE GRANT
Subject to the terms and conditions of this Agreement, CSIC hereby grants a worldwide, non-exclusive, nontransferable, licence to MPP, under the Licensed Technology, to grant sublicences to Sublicensees selected by MPP and WHO C-TAP to:
a) Develop, or have developed, the Licensed Technology into Products in the Field, and
b) Make, have made, use, Commercialize, export or import the Products exclusively for ultimate use in the Field.
For the avoidance of doubt, this Agreement does not grant any rights in relation to Biofabri Material and Know-how.
4. TERRITORIAL SCOPE
The license under this Agreement is granted worldwide.
8. ASSIGNMENT AND SUBLICENSES
[…]
8.2. Licences and sublicences
MPP and CSIC will discuss and agree upon the identities of interested and suitable Third Parties to whom MPP shall grant sublicences for the purposes of developing, fabricating and/or Commercialising the Product. MPP will require in the sublicences that sublicensee(s) use commercially reasonable efforts to ensure that the Product(s) be made available in LMICs at affordable pricing.
11. CONSIDERATIONS AND FOLLOW-UP REPORTS
As consideration for the rights conveyed by CSIC under this Agreement, MPP shall use reasonable efforts to sublicense the rights to develop, use and Commercialize the Patent Rights and Material to companies interested to manufacture and/or Commercialise the Product. MPP will keep CSIC regularly informed of the progress in the search for sublicensees.
Schedule 2: The Licensed Patents
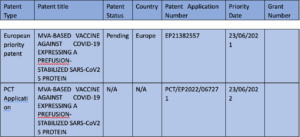
Schedule 3: Licensed Know-how and Materials
a) Materials: Premaster virus seed for MVA-CoV2-S(3P) and derived materials, and any other Materials useful for the manufacturing and/or development of the Product owned and/or controlled by CSIC.
b) Licensed Know-how: as of the Effective Date is mostly related to scientific background and preclinical development of MVA-based COVID-19 vaccine candidate, MVA-CoV2-S(3P). Transfer of the Licensed Know-how will consist of the following items:
- Technical support:
- Plant visits and training: training of Sublicensee technical engineers, at, as the case may be, the Sublicensee’s facilities or CSIC facilities that are developing or using the licensed process and/or making and selling the Product.
- Direct assistance: qualified and experienced professional from or on behalf of CSIC to advise the Sublicensee on the use of Licensed Know-how for manufacture of the Products.
- Consultation: Sublicensee shall have the right to contact CSIC by mail or telephone through representatives appointed by each party in relation to the use of Licensed Know-how, including without limitation for any quality and regulatory questions.
- Timeline: each transfer shall be performed directly to the Sublicensee in the shortest time possible and in any case within 60 days from MPP’s request.
