MAPGuideⓇ Commentaries
GHIAA’s comments on the Resumed INB 9 Proposal for the WHO Pandemic Agreement
On April 22, 2024, the WHO published a copy of the updated Proposal for the WHO Pandemic Agreement, which will be discussed during the resumed INB 9 negotiations taking place from April 29 – May 10, 2024. This latest text follows the previous Revised Draft (March 2023), Negotiating Text (October 2023), Bureau’s Text (May 2023), and Zero Draft (February 2023).
GHIAA’s comments and recommendations below focus on the Proposal provisions related to the use of public funds for the development of pandemic-related health products. We highlight areas in which commitments to facilitate equitable access to these products should be strengthened or reinstated, and outline some initial considerations for national governments as they begin to assess the actions needed to implement the Agreement post-adoption.
Agreement Conditions for Publicly Funded R&D
Extracts from the Proposal (emphasis added throughout)
(d) “pandemic-related health products” means the safe, effective, quality and affordable products that are needed for pandemic prevention, preparedness and response, which may include, without limitation, diagnostics, therapeutics, vaccines and personal protective equipment
1. The Parties shall cooperate to build, strengthen and sustain geographically diverse capacities and institutions for research and development, particularly in developing countries, based on a shared agenda, and shall promote research collaboration and access to research through open science approaches for the rapid sharing of information and results, especially during pandemics.
[…]
3. The Parties shall, in accordance with national circumstances and mindful of relevant international standards and obligations, take steps to strengthen international coordination and collaboration to support well-designed and well-implemented clinical trials, by developing, strengthening and sustaining clinical trial capacities and research networks, at the national, regional and international levels, and facilitating the rapid reporting and interpretation of data from such trials.
4. Each Party shall ensure that government-funded research and development agreements for development of pandemic-related health products include, as appropriate, provisions that promote timely and equitable access to such products and shall publish the relevant terms. Such provisions may include: (i) licensing and/or sublicensing, preferably on a non-exclusive basis; (ii) affordable pricing policies; (iii) technology transfer on mutually agreed terms; (iv) publication of relevant information on research inputs and outputs; and/or (v) adherence to product allocation frameworks adopted by WHO.
Comments & Recommendations
1. We are encouraged that commitments under Article 9.4 related to equitable access conditions for government funded R&D have been retained from the Revised Draft. We note that there have been some constructive edits to the previous text, in particular:
- We welcome the removal of the restrictive language from the Revised Draft which stated that provisions should promote equitable access “during public health emergencies of international concern and pandemics.” The removal of this restriction recognizes the importance of access to pandemic-related health products to prepare for and prevent public health emergencies, not just respond to them. It also aligns with the definition of “pandemic-related health products” included in the Proposal which covers products for pandemic prevention, preparedness, and response.
- We further welcome the addition of the commitment to “ensure” that equitable access provisions are included in government-funded R&D agreements.
2. We note that some elements of Article 9.4 would benefit from further strengthening, in particular:
- The Proposal text refers to “government funded” R&D, which risks excluding some types of indirect government R&D funding from the commitments under Article 9.4. For example, public research institutions are run with government funding and may be responsible for granting R&D funding to third parties, but they are not necessarily categorized as government agencies or departments.
Recommendation: the term “government funded” should be replaced with “publicly funded”.
Considerations for implementation: extension of the commitments under Article 9.4 to R&D funding granted through indirect or intermediary funding streams can be approached using a two step process:
- First, national governments need to attach requirements to the initial direct grant or allocation of government funds to intermediary agencies and/or institutions. These requirements could be established through national policies or regulations, agreement provisions, and/or similar mechanisms, and should stipulate that any onward flow of funding to a third party to support product R&D must be subject to a written agreement including equitable access obligations.
- Second, intermediary agencies should implement policies, procedures, and agreement provisions to ensure compliance with the national government requirements.
- The Proposal text states that publicly funded R&D agreements will “include, as appropriate, provisions that promote timely and equitable access.” While provisions should be appropriate to the context of a particular agreement, this language is open to interpretation, leaving room to exclude access provisions if they are not considered “appropriate.”
Recommendation: the text should be amended to clarify the expectation for equitable access provisions to be included in all public R&D funding agreements. For example: “The Parties will ensure that publicly-funded R&D agreements for pandemic-related health products include provisions designed to facilitate equitable access to products and other results arising from public funding. Such provisions will be drafted as appropriate on an agreement-by-agreement basis and may include, but are not limited to …”
Useful resource: GHIAA’s Equitable Access Toolkit provides examples of equitable access provisions included in existing R&D funding agreements. These examples illustrate a range of requirements that can be considered based on factors such as product type and development stage.
- Article 9.4 includes a commitment from the Parties to publish “relevant terms” from publicly funded R&D agreements. This language leaves the judgment of “relevance” at the discretion of the Parties and risks the publication of minimal agreement excerpts taken out of context thereby minimizing opportunity to hold the Parties and funded entities accountable to their commitments.
Recommendation: The Agreement should include a requirement for the Parties to publish the terms of publicly funded R&D agreements through an easily discoverable and accessible route, with minimal and fully justified redactions.
- We are pleased that the Proposal text has retained examples of the types of equitable access provisions that could be included in publicly funded R&D agreements. However, the examples may benefit from some clarification to ensure that they can be implemented effectively:
- 9.4(i) licensing & sublicensing and 9.4(iii) technology transfer – from a practical perspective, it is important to recognize that licensing and a commitment to technology transfer often need to be undertaken together. On one hand, technology transfer is often accompanied by a license to use the related intellectual property (IP); on the other, a license may be of limited use without an accompanying technology transfer (although this is dependent on the technology in question). It is also important to identify the expected recipients and purpose of the licenses and/or technology transfer.
Recommendation: the Agreement should specify that licensing and technology transfer of IP generated through publicly funded R&D agreements should be made to geographically-diversified manufacturers and/or WHO-coordinated hubs – both of which are considered in more detail in Articles 10 & 11 – on reasonable terms, for the purpose of ensuring sufficient and timely availability of safe and affordable products.
Useful resource: GHIAA’s commentary on IP rights management provisions provides examples of licensing and technology transfer requirements in existing R&D funding agreements.
- 9.4(ii) affordable pricing policies – R&D funding agreements usually contain affordable pricing obligations or commitments, rather than policies.
Recommendation: the Agreement could refer to affordable pricing “commitments” rather than “policies” to indicate clear expectations that products developed using public funding will be made affordable, rather than just developing policies for affordable pricing (which may or may not be implemented).
Considerations for implementation: when establishing affordable pricing commitments in their agreements, public funding agencies should work with funded developers and procurement agencies to assess how best to balance affordability against the ongoing financing necessary to ensure long-term availability of the product.
Useful resource: GHIAA’s commentary on Strategies for Affordable Pricing Provisions in R&D funding agreements provides examples of different approaches to affordable pricing commitments, including consideration of sustainability.
- 9.4(iv) publication of relevant information on research inputs and outputs – use of the terminology “research inputs and outputs” differs from terminology commonly found in R&D existing funding agreements, which tend to refer to “open access,” “open science,” and the sharing of clinical trial data and results.
Recommendation: the Agreement may benefit from more consistent use of terminology to better connect the commitments made under Article 9. For example, the commitments to promote “open science” approaches under Article 9.1, and to facilitate rapid reporting of clinical trial data under Article 9.3 should be carried through to the provisions included in publicly funded R&D agreement provisions under Article 9.4.
Useful resource: GHIAA’s commentary on Data Sharing and Publication Provisions provides examples of the approaches and terminology used in existing R&D funding agreements.
- 9.4(v) adherence to product allocation frameworks adopted by WHO – adherence to product allocation frameworks is one commitment that could facilitate the equitable availability of publicly funded pandemic-related health products. However, it is unlikely that this commitment alone will be sufficient to ensure that such products are available when and where they are needed.
Recommendation: Publicly funded R&D agreements should include commitments to ensure that pandemic-related health products are registered in the countries where they are needed, as well as to make those products available for purchase by relevant purchasing agencies in accordance with any product allocation frameworks or mechanisms facilitated by the Global Supply Chain and Logistics Network established under Article 13.
Considerations for implementation: ensuring global product availability is a complex challenge requiring navigation of multiple regulatory pathways in multiple jurisdictions, as well as the establishment of substantial supply chain, manufacturing, and distribution capacity and networks. Licensing and technology transfer could play a crucial role in addressing this challenge by enabling the establishment of increased and geographically diversified manufacturing capacity with facilities that can leverage their knowledge of local and regional regulatory systems to facilitate product registration.
- Additional considerations for Article 9.4
- Equitable access provisions included in publicly funded R&D agreements may fail to achieve their intended impact if a funded developer is unwilling or unable to fulfill its obligations. It is therefore important for public funding agencies to ensure that their funding agreements include enforcement provisions that allow the agency to access publicly funded IP so that it can be used for its intended purpose. R&D funding agreements often include an “access license” (also referred to as “step-in rights”, a “humanitarian license” or a “public health license”) that enables a funder to continue product development and commercialization with a new partner.
- Additional considerations for Article 9.4
Useful resource: Examples of Access License provisions, and discussion of the associated triggers, rights and obligations are available here.
- The Agreement does not include any reference to the development and monitoring of access plans for pandemic health-related products. These can be a valuable tool for considering how product affordability, availability, accessibility, adoption and sustainability can be addressed. Requirements to develop access plans can be included in early stage R&D funding agreements and evolve alongside product development and commercialization plans.
- Public R&D funding agreements are often granted early on in the R&D process. The funded product developer is likely to pass through a portion of that funding, and/or the IP generated with that funding, to third parties. Funding agreements will therefore need to include “flow through” obligations to ensure that equitable access obligations remain attached to the public funding and resulting IP.
Useful resource: examples of relevant provisions from existing R&D funding agreements are available here.
3. We note the removal of language from the Revised Text related to the development of national policies for the inclusion of equitable access provisions in publicly funded R&D agreements.
Considerations for implementation: regardless of whether requirements to develop national policies are included in the Agreement, national governments and public funding agencies will need to consider the development and implementation of regulations, policies, procedures and/or practices that facilitate and monitor the fulfillment of the commitments made under Article 9.4.
Licensing and Technology Transfer to Support Geographically Diversified Production
Extracts from the Proposal (emphasis added throughout)
1. The Parties commit to achieving more equitable geographical distribution and scaling up of the global production of pandemic-related health products, and increasing sustainable, timely, fair and equitable access to such products, as well as reducing the potential gap between supply and demand during pandemics, through transfer of relevant technology and know-how on mutually agreed terms.
2. The Parties, in collaboration with WHO and other relevant organizations, shall:
a) Take measures to provide support for, maintain and/or strengthen, as appropriate, facilities at national and regional levels, particularly in developing countries, and those that have conducted disease burden studies relevant to pathogens with pandemic potential, with a view to promoting the sustainability of such investments, for the production, or scaling up of production, of relevant pandemic-related health products;
b) take measures, in accordance with national and/or domestic laws, as appropriate, and regulations, to identify and contract with manufacturers other than those referenced in paragraph 2(a) of this Article, for scaling up the production of pandemic-related health products, during pandemics, in cases where the production and supply capacity of the production facilities does not meet demand;
c) actively support, participate in and/or implement, as appropriate, relevant WHO technology, skills and know-how transfer programmes to facilitate strategically and geographically distributed production of pandemic-related health products;
[…]
1. Each Party shall, in order to enable sufficient, sustainable and geographically-diversified production of pandemic-related health products, and taking into account its national circumstances:
a) promote and otherwise facilitate or incentivize the transfer of technology and know-how for pandemic-related health products, in particular for the benefit of developing countries and for technologies that have received public funding for their development, through a variety of measures such as licensing, on mutually agreed terms;
b) publish the terms of its licenses for pandemic-related health technologies in a timely manner and in accordance with applicable law, and shall encourage private rights holders to do the same;
c) encourage research and development institutes and manufacturers, in particular those receiving significant public financing, to forgo or reduce, for a limited duration, royalties on the use of their technology for the production of pandemic-related health products;
d) promote the transfer of relevant technology and related know-how for pandemic-related health products, by private rights holders, on fair and most favourable terms, including on concessional and preferential terms and in accordance with mutually agreed terms and conditions, to established regional or global technology transfer hubs or other multilateral mechanisms or networks, as well as the publication of the terms of such agreements;
e) encourage holders of relevant patents that received public funding, and where appropriate, other holders of relevant patents for pandemic-related health products, to forgo royalties or otherwise license any relevant patents at reasonable royalties to developing country manufacturers for the use, during the pandemic, of their technology and know-how for the production of pandemic-related health products;
[…]
Comments & Recommendations
1. We welcome the addition of the language “through transfer of relevant technology and know-how on mutually agreed terms” to the end of Article 10.1. This clearly connects the commitments outlined under Articles 10 and 11 with the goal of achieving equitable access.
2. Article 10.2(a) appears to aim to establish sustainable national and regional manufacturing facilities for the production of pandemic-related health products. Article 10.2(b) then refers to the contracting of additional manufacturers for scaling-up production during pandemics if the facilities established under 10.2(a) cannot meet demand.
Considerations for implementation: the process required to contract additional manufacturers under Article 10.2(b) may depend on the type of product. For example, it may be reasonably straightforward to contract back-up capacity for products that have relatively uncomplicated supply chains and IP considerations. However, it could be substantially more complex to engage a contract manufacturer to produce a vaccine owned by a third party; this would require multiple agreements and collaborations with multiple parties to secure the IP rights, technology transfer and raw materials for the vaccine in question.
It is also important to consider the timing of any agreements intended to increase production and supply capacity. Assessment of requirements for capacity reservation agreements should be approached as a pandemic preparedness activity, rather than waiting for a shortage to occur. Some governments may have powers to requisition capacity in certain circumstances, but this is unlikely to be the most efficient approach to ensuring timely supply.
3. Article 11(a) refers to “promoting”, “facilitating” or “incentivizing” technology and know-how transfer, particularly for technologies “that have received public funding for their development.” This language is vague and could potentially include both (i) technology generated under the R&D funding agreements addressed in Article 9, and (ii) technology generated by public research institutions and owned by the government. However, an article specifically addressing licensing of government-owned IP that was included in the previous Revised Draft has been removed from the current Proposal, although reference to publication of the terms of such licenses remains under Article 11.1(b).
Government-owned IP can play a substantial role in the development of pandemic-related health products. For example, licenses from the U.S. Department of Health and Human Service (HHS) were used in the development of a number of vaccines and therapeutics, including the Moderna and BioNTech/Pfizer COVID-19 vaccines. It is therefore essential that the Parties make explicit commitments to share this IP in line with their commitments under Articles 10.1, 10.2(c) and 11.1.
Recommendation: as illustrated by the diagram below, the Agreement should include specific commitments both to include licensing and technology transfer obligations in publicly-funded R&D agreements (as addressed under Article 9), and for licensing and technology transfer of government-owned IP (under Article 11).
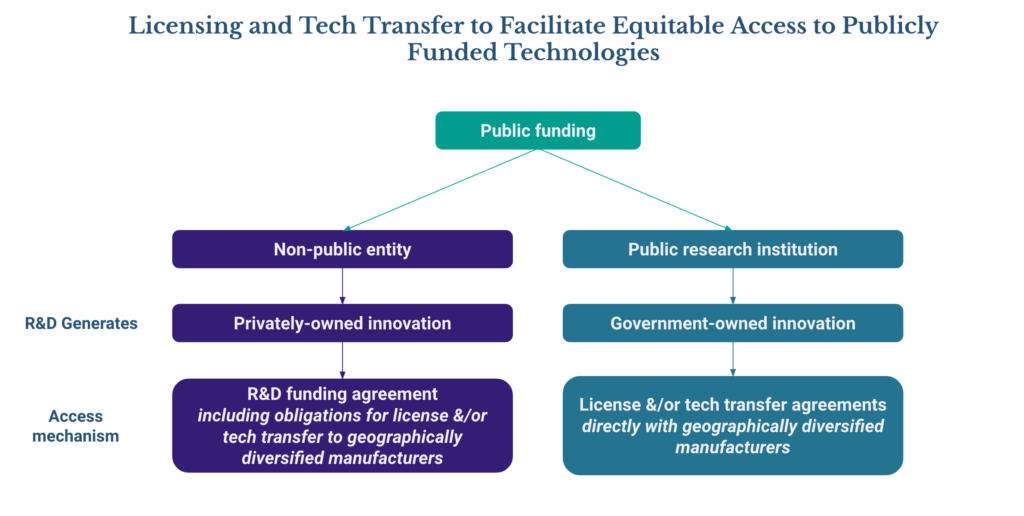
Considerations for implementation: Considerations for implementation: to maximize the possibilities for facilitating equitable access through licensing and technology transfer, agreements should ideally be on non-exclusive or restricted exclusivity terms (e.g., limited to certain fields of use or territories). Any exclusive licenses should include equitable access obligations as well as reservation of rights, reversion of rights and/or required sublicensing provisions that enable another party to develop and commercialize the technology if the exclusive licensee is unable or unwilling to meet equitable access requirements.
For licensing of government-owned innovations, it is likely that national governments and public research institutions will need to develop and implement regulations, policies, procedures, and/or practices that consider how to best facilitate and monitor the achievement of equitable access through agreement provisions.
4. Articles 11.1(c) and 11.1(e) refer to “encouraging” R&D institutes, manufacturers and patent holders – particularly those that have received public funding – to forgo or reduce royalties on use of their patents & technologies. We reiterate our previous comments that requirements for waived, reduced, or tiered royalties in certain circumstances should be included in the equitable access conditions for publicly-funded R&D agreements under Article 9.4. It is also not clear why there are separate provisions related to encouraging R&D institutes and manufacturers to forgo or reduce royalties on use of their technologies (Article 11.1(c)) and encouraging patent holders to forgo or reduce royalties on use of their patents (Article 11.1(e)).
Considerations for implementation: reduced or waived royalties can support affordable pricing by reducing a licensee’s total cost of production. However, the licensor–for example, a publicly-funded developer–incurs human resource and other costs when conducting a technology transfer. They may also have incurred R&D costs related to the technology in question that are not covered by public funding. Appropriate compensation for these costs is important for the sustainability of the product developer’s own activities. The relevant parties will need to consider suitable compensation mechanisms based on specific contexts; these could include profit margins from sales made to higher income markets, public R&D funding, royalties under a licensing agreement, and/or through funding under capacity development programs.
Taking the Opportunity to Ensure Coordinated and Comprehensive Commitments
This commentary has identified a number of areas where the connections between Articles 9, 10 & 11 need to be more clearly considered and referenced in the Agreement text to support efficient and effective implementation by the Parties. The diagram below highlights some key connections for facilitating equitable access to publicly funded technologies including:
- Commitments made by the parties across Articles 9, 10, and 11 to develop and strengthen geographically diversified manufacturing capacity that is able to receive technology transfer, with the objective of achieving equitable access to pandemic-related health products.
- Access conditions attached public R&D funding agreements under Article 9 which are then fulfilled by the publicly funded developer by: (i) directly ensuring the timely availability of affordable products; and (ii) licensing and technology transfer to geographically diversified manufacturers under reasonable royalty terms in support of the objectives of Articles 10 and 11; and
- Use of public funds to develop government-owned innovations which are then licensed to geographically diversified manufacturers as part of Parties’ fulfillment of commitments made under Articles 10 & 11.
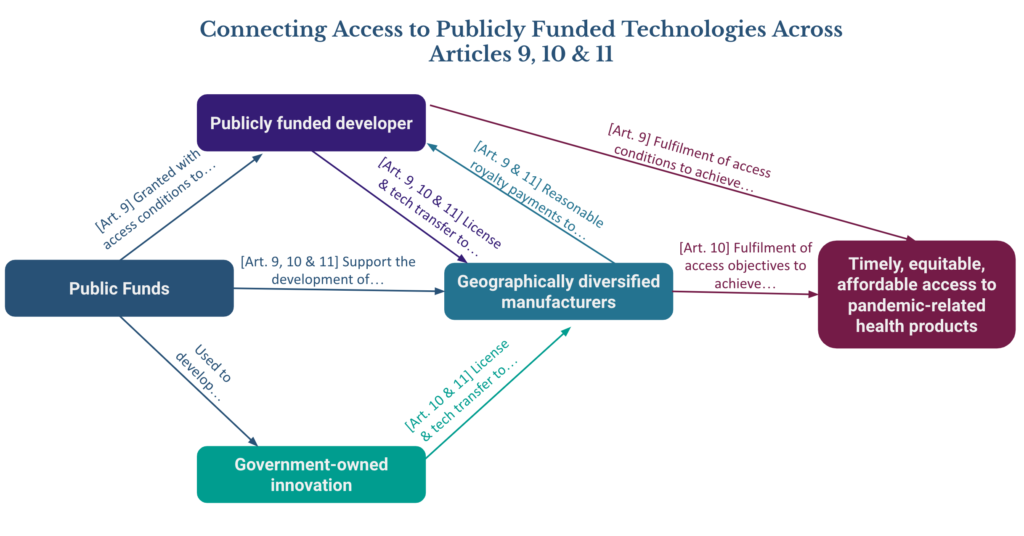
In line with our comments on previous draft texts for the Agreement, we continue to encourage timely, proactive, and open publication of the agreements included in the diagram above, as well as the resulting procurement agreements. This level of transparency will support monitoring and accountability mechanisms for all parties, and provide an opportunity to build trust and equal access to information.
With the resumed INB 9 negotiations expected to be the final round of discussions prior to presentation of the Agreement at the World Health Assembly in May, we urge INB negotiators to to consider the collective comments and recommendations made above and to take the opportunity to strengthen commitments to achieving equitable access to pandemic-related health products.
Authors: Bridie Telford
First Publication Date: April 24, 2024
