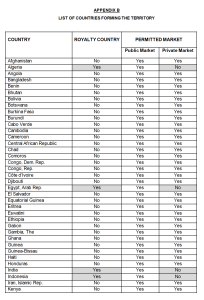
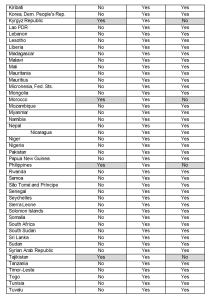
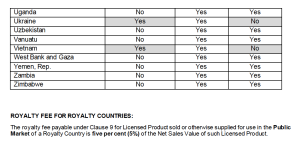
Provision Language
Definitions
“Private Market” means any entity in any country in the Territory that is not in the Public Market.
“Public Market” means:
(A) the following organisations to the extent that they are not for profit organisations:
(i) the government of any country in the Territory, including the ministries and agencies of such government, appointed procurement agencies acting on behalf of such government, and institutions and programs funded by such government such as state-run hospitals and prison services (referred to together as “Government”);
(ii) non-government organisations (“NGOs”) recognized by the applicable Government;
(iii) organisations of the United Nation working for or in a country of the Territory, including but not limited to UNDP and UNICEF;
(iv) Médecins Sans Frontieres, Save the Children, OXFAM and the International Committee of the Red Cross (ICRC); and
(v) the funding mechanisms (and programs funded by such mechanisms) Unitaid, PEPFAR, U.S. Agency for International Development, Children’s Investment Fund Foundation or Global Fund, or procurement agencies acting on their behalf, to the extent that they support local implementation of public HIV prevention program(s) for people >35 kg at risk of HIV infection in a country of the Territory; and
(B) Third Party distributors, solely to the extent that such Third Party distributors distribute the Licensed Product to or for one or more entities identified in (A) […].
“Relevant Regulatory Authority” means (i) in relation to a particular country in the Territory, any applicable federal, national, regional, state, provincial, or local regulatory agencies, departments, bureaus, commissions, councils or other government entities regulating or otherwise exercising authority with respect to the Licensed Product in that country, or (ii) WHO pre-qualification programme where such approval has been deemed adequate by the authority referred to in (i).
“Regulatory Approval” means, in relation to each country of the Territory and each Licensed Product, a marketing authorisation from a Relevant Regulatory Authority for that Licensed Product for use in the Field in that country.
“Usage Period” means, in relation to each proposed sale or supply, the calendar period within which the Licensed Product subject to such proposed sale or supply is intended to be used, in each instance as indicated by the relevant Permitted Market.
7. Approval Requests
The Parties acknowledge and agree that pursuant to the terms of the Sublicence, each Sublicensee is required to seek the MPPF’s approval in respect of certain actions. The MPPF shall, using reasonable skill and care, promptly review:
7.1 Royalty Country Public Procurement – under Clause 3.6 of the Sublicence, any request by a Sublicensee for an Approved Royalty Country Public Procurement. The MPPF shall determine based on the evidence provided by the Sublicensee, whether the proposed sale or supply is (i) to a Public Market and (ii) of a volume of Licensed Product that is commensurate with the demand for Licensed Product(s) in such Public Market for the applicable Usage Period, as such demand is reasonably estimated by the MPPF. The MPPF shall only approve (or allow its approval to be deemed to be granted) for proposed sales or supplies that are to a Public Market and of a volume of Licensed Product(s) that is commensurate with demand;
8. Sublicence Compliance
8.1 Monitoring – The MPPF shall, using all reasonable care, diligence and skill, actively monitor each Sublicensee’s compliance with the terms of its Sublicence, including:
[…]
(C) Non-diversion – verifying that:
(i) all sales and/or supplies of Licensed Product by each Sublicensee were made in compliance with Clause 10 of the Sublicence; and
(ii) the volume of Licensed Product sold and/or supplied by Sublicensee(s) for use in any Permitted Market for any period of time is commensurate with the demand for Licenced Product(s) in such Permitted Market for such period of time, as reasonably estimated by the MPPF. If the volume of Licenced Product sold and/or supplied by Sublicensee(s) for use in a Permitted Market exceeds such demand, the MPPF shall take reasonable steps to determine whether any Sublicensee has breached the terms of its Sublicence;
Schedule 2 – Form of Sublicense
3. GRANT OF LICENCE
3.6 Royalty Country Public Procurements. In any Royalty Country, the Licensee must obtain prior written approval from the Licensor for any sale or supply of Licensed Product by the Licensee in the Public Market, unless the Licensor has expressly indicated that approval is not granted. The Licensor shall approve or reject any such written requests within five (5) Business Days of receipt of the request and approval shall be deemed given if not issued by the end of the fifth Business Day following receipt of the request. Any such written request for approval shall include copies of the relevant Royalty Country procurement documentation regarding the proposed sale or supply. Any Royalty Country Public Market procurement approved by the Licensor pursuant to this Clause 3.6 shall be referred to in this Agreement as an “Approved Royalty Country Public Procurement”.
4. DEVELOPMENT, REGISTRATION AND COMMERCIALISATION
[…]
4.3 Timelines for Regulatory Approval. The Licensee shall file, for Regulatory Approval (for both an oral Licensed Product and an extended-release Licensed Product) before at least one Relevant Regulatory Authority as soon as possible and in any event not later than 60 months from the Effective Date, in each case using the fastest approval route possible. Licensee shall also, upon Licensor’s reasonable request, file for regulatory approval before the Relevant Regulatory Authority for any subsequent Licensed Product within a reasonable time.
[…]
4.5 Access. The Licensee shall, acting in compliance with all Applicable Law, use its best endeavours to enable broad access to Licensed Product in the Permitted Market of a country of the Territory as soon as it has obtained Regulatory Approval for such Licensed Product in such country.
10. NON-DIVERSION
10.1 Restrictions. Save as provided under this Agreement and without prejudice to Clause 3.5, and to the extent that such restrictions comply with Applicable Law, the Licensee shall not:
10.1.1 register, manufacture, offer for sale, sell or otherwise supply Licensed Product for use outside the Permitted Market; or
10.1.2 offer for sale, sell or otherwise supply Licensed Product for use outside the Field, unless Licensee has prior written consent from the Licensor; or
10.1.3 offer for sale, sell or otherwise supply Licensed Product to any Third Party that the Licensee knows, believes or ought reasonably to suspect will sell or supply the Licensed Product for the uses set out in Clauses 10.1.1 and 10.1.2 above;or
10.1.4 offer for sale, sell or otherwise supply any Licensed Compound to any Third Party other than a Cabotegravir Licensee for use under a Cabotegravir Licence.
10.2 Compliance measures. The Licensee shall use reasonable efforts to ensure its compliance, and compliance by any Third Party to which it sells or supplies Licensed Product, with the terms of this Clause 10, including implementing the following measures:
10.2.1 Prior to any sale or supply of Licensed Product, the Licensee shall give written notice to a Third Party to which it intends to sell or supply Licensed Product of the restrictions contained in this Clause 10, and except where such sale or supply is made directly by the Licensee to a relevant Government (as defined under Clause 1.48), and without prejudice to its obligations under Clause 10.1.3, the Licensee shall obtain written undertakings from such Third Party that the Third Party will sell, supply and/or use the Licensed Product in compliance with the restrictions imposed by this Agreement, including the restrictions regarding Permitted Market and Field;
10.2.2 The Licensee shall assist the Licensor to secure compliance by a Third Party to which it has sold or supplied Licensed Product with this Clause 10 and the restrictions which it contemplates;
10.2.3 The Licensee shall maintain a quick and efficient batch trace procedure following the GS1 Global Traceability or comparable standards so as to enable the identification and location of Licensed Product from individual batches with minimal delay;
10.2.4 The Licensee shall implement the batch trace procedure referred to in Clause 10.2.3 at the request of the Licensor if at any time the Licensor is of the opinion that any batch or batches of the Licensed Product have been, or may have been, diverted outside the Permitted Market, or Field; and
10.2.5 The Licensee shall ensure, before each sale or supply of Licensed Product, that the volume of Licensed Product it intends to sell or supply is commensurate with the demand for Licensed Product for the proposed Usage Period in the Permitted Market, as such demand is reasonably estimated by the Licensee. Where the volume of Licensed Product to be sold or supplied exceeds such demand (as reasonably estimated by the Licensee), the Licensee shall take all reasonable steps to ensure that the relevant sale or supply will not breach the terms of this Agreement, including the restrictions regarding Permitted Market.
10.3 Breach. Without prejudice to the Licensor’s rights under Clause 21, if at any time the Licensee becomes aware that it, or a Third Party to which it has sold or supplied Licensed Product, has sold or supplied Licensed Product in breach of the terms of this Clause 10, the Licensee shall:
10.3.1 immediately notify the Licensor in writing, providing details of such breach; and
10.3.2 provide to the Licensor, within thirty (30) days of such notification, details of a mitigation plan to prevent any repeated sale or supply in breach of this Agreement.