Provision Language
Definitions
“Additional ARV” means anti-retroviral active pharmaceutical ingredients used in the treatment of HIV and consisting of (listed alphabetically): abacavir, dolutegravir, emtricitabine, lamivudine, tenofovir disoproxil fumarate and tenofovir alafenamide.
“Agreed ARV” means an anti-retroviral active pharmaceutical ingredients used in the treatment of HIV consisting of (listed alphabetically): atazanavir, cobicistat, darunavir, doravirine, efavirenz, lopinavir (including ritonavir-boosted lopinavir), raltegravir, and ritonavir, and combinations thereof.
“ARV-SDN” means an Agreed ARV and/ or Additional ARVs, or combinations thereof, prepared as solid drug nanoparticles using the Licensed Technology and suitable for formulation into a pharmaceutical product.
“Licensed Know-how” means all technical information or know-how known to or controlled by the University as of the Effective Date (including all manufacturing data, the percentages and specifications of ingredients, the manufacturing process, specifications, assays, quality control and testing procedures) that is identified by the University as primarily and directly relating to, and reasonably necessary for, the making of an ARV-SDN or a Licensed Product in the same manner that such ARV-SON or licensed Product has been made by the University prior to the Effective Date, as well as any other modification or improvement of such technical information or know-how known to or controlled by the University and unencumbered after the Effective Date.
“Licensed Patents” means the solid drug nanoparticle technology as described in the patents and patent applications set out in Schedule 1, as may be amended from time to time, including any continuations, continuations in part, extensions, reissues, divisions, and any supplementary protection certificates and similar rights deriving priority from any of these.
“Licensed Product” means any pharmaceutical product which entirely or partially uses the Licensed Technology in either its development, manufacture, regulatory approval or whose manufacture, use or sale would constitute an infringement of any patent claim within the Licensed Technology.
“Licensed Technology” means the Licensed Patents and the Licensed Know-how;
2. DEVELOPMENT OBLIGATIONS
[…]
2.3 MPP will use its best endeavours to identify and enter into agreements with Development Partner(s) that can assist in developing ARV-SDNs into Licensed Products, file them for regulatory approval and transfer technology to MPP’s Commercialisation Partner(s).
[…]
2.7 MPP will use its best endeavours to identify Commercialization Partner(s) capable of commercially exploiting the Licensed Technology and any Licensed Products in accordance with the terms of this Agreement.
3. GRANT OF LICENCE AND RESERVATION OF RIGHTS
3.1. Subject to the terms of this Agreement and with effect from the Effective Date, the University grants to MPP:
3.1.1. a non-exclusive, non-transferable worldwide licence under the Licensed Technology to grant sub-licences, in accordance with the terms set forth in Schedule 4, to Development Partner(s) to develop, or have developed, ARV-SDNs into Licensed Products in the Field; and
3.1.2. a non-exclusive, non-transferable, royalty-bearing worldwide licence under the Licensed Technology to grant sub-licences, in accordance with the terms set forth in Schedule 5, to Commercialisation Partner(s) to make, have made, use, offer for sale, sell, have sold, export or import the Licensed Products in the Field exclusively for administration to patients in the Territory.
3.2. For the avoidance of doubt no rights are afforded to MPP under this Agreement for MPP or MPP Licensees to:
3.2.1. create ARV-SDNs independently of the University; or
3.2.2. use the Licensed Technology outside the Field; or
3.2.3. offer for sale, sell, have sold or otherwise commercialise Licensed Products for use outside the Territory; or
3.2.4. have any right, title or interest to any intellectual property unless otherwise explicitly stated; or
3.2.5. in the case of MPP, to directly practice such licences or otherwise exploit the Licensed Technology for any other purpose other than to grant sub-licences under Clause 3.1.1 and 3.1.2 of this Agreement.
3.3. Notwithstanding anything contained in this Agreement, it shall not be a breach of this Agreement for MPP, or MPP Licensees, to:
3.3.1. supply to a country where a compulsory licence has been issued by the government of such country or;
3.3.2. conduct any activities where such activities would not infringe a Licensed Patent granted and in force, or do not rely on Licensed Know-How.
3.4. After the first anniversary of this Agreement, the Parties may agree to explore an extension to the Field to include other therapeutic areas such as malaria, tuberculosis, hepatitis B and hepatitis C. In the event that an agreement is reached, any such Field Extension shall be executed as a separate written amendment to this Agreement.
4. RIGHT TO SUB-LICENSE TO MPP LICENSEES
4.1. MPP may grant sub-licences and may disclose to MPP Licensees only such of the Confidential Information as is necessary for the exercise of the rights sub-licensed, subject in each case to the following conditions:
4.1.1. MPP and the University mutually agree suitable MPP Licensees within 30 days of MPP proposing a potential MPP Licensee, a potential MPP Licensee is deemed to be mutually agreed unless otherwise noted in writing by the University stating the grounds and the mitigation approach; and
4.1.2. MPP provides the University with a copy of each Sub-Licence Agreement together with a summary of the same, within 10 days after its grant; and
4.1.3. the MPP Licensee accepts obligations and conditions consistent with those in this Agreement including Schedule 4 and Schedule 5 (as applicable); and
4.1.4. MPP will ensure that the Sub-Licence Agreement(s) will grant to the University a non exclusive, perpetual, worldwide, royalty-free license to use any Improvement; and
4.1.5. MPP will ensure that Sub-licence Agreements contain indemnifying obligations against any direct loss, damages, costs, claims or expenses which are awarded against or suffered by the University, its officers, employees, sub-contractors and agents as a result of any act or omission of the MPP Licensee.
4.1.6. University will have the right under the Contracts (Rights of Third Parties) Act 1999 to enforce and rely on the terms of the Sub-licence Agreement(s).
4.2 MPP agrees to monitor compliance of each MPP Licensee. Such monitoring shall include:
4.2.1 reviewing with all reasonable skill and care any reports provided to MPP by the MPP Licensee under the relevant sections of the Sub-Licence Agreement;
4.2.2 fully exercising the audit right set out in the Sub-Licence Agreement(s) as soon as MPP has reasonable cause to believe (or as soon as University and MPP have agreed that they have reasonable cause to believe) an audit is necessary.
4.3 If the MPP becomes aware of any act or omission of an MPP Licensee which constitutes a breach of the relevant Sub-Licence Agreement, the MPP shall notify the University immediately and (i) if the breach is capable of correction and does not give rise to an immediate right of termination under the Sub-Licence Agreement, direct the relevant MPP Licensee in writing to cure the breach, with a simultaneous copy of that writing to University; and (ii) if the breach remains uncured at the end of the specified period, or if there are otherwise grounds for termination under the Sub-Licence Agreement, and in each case if so requested by University, procure the termination of the relevant Sub-Licence Agreement in accordance with its terms.
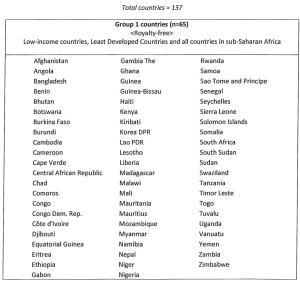
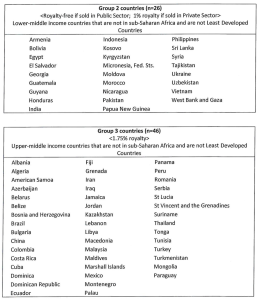
Schedule 3 – The Territories
Schedule 4 – Development Agreement Term Sheet
1. Scope of the grant: MPP will grant a non-exclusive, non-transferable worldwide licence under the Licensed Technology to allow Development Partners to develop, or have developed, ARV SDNs into Licensed Products in the Field. For the avoidance of doubt no rights are afforded that allow the Development Partner to create new ARV-SDNs or undertake any Licensed Product sales activities.
MPP will require that the Development Partner to perform the following activities:
(i) Scaling up manufacturing of ARV-SDN at industrial scale
(ii) Conduct formulation development of ARV-SDNs into Licensed Products in the Field, under oversight of a Steering Committee (as defined below).
For the avoidance of doubt Development Partner will be expressly prohibited from further sub-licensing the Licensed Technology to any other third party.
5. Grant-back rights: Development Partner will grant to MPP and University a perpetual, irrevocable, worldwide, royalty-free, non-exclusive, sub-licensable licence over any Improvement (and shall promptly execute such document as University may reasonably request accordingly). Such licence will not affect the Licensee’s ownership of the Improvements. MPP will have the right to sub-license such rights to its Commercialisation Partner(s).
Schedule 5 – Commercialisation Agreement Term Sheet
1. Scope of the grant: MPP will grant a Commercialization Partner a non-exclusive, non transferable, royalty-bearing worldwide licence under the Licensed Technology to allow Commercialization Partners to make, use, offer for sale, sell, export and import the Licensed Products for the purposes of commercialising Licensed Products in the Field solely for use within the Territory.
For the avoidance of doubt Commercialisation Partner will be expressly prohibited from further sub-licensing the Licensed Technology to any other third parties and from making sales of Licensed Products for use outside the Territory.
[…]
6. Grant-back rights: Commercialisation Partner will grant to MPP and University a perpetual, irrevocable, worldwide, royalty-free, non-exclusive, sub-licensable licence over any Improvement (and shall promptly execute such document as University may reasonably request accordingly). Such licence will not affect the Commercialisation Partner’s ownership of the Improvements. Commercialisation Partner will agree to engage in good-faith negotiations should MPP desire to further sublicense such Improvements. University shall be entitled to grant sub-licences (without further right to sublicense) to other third parties, provided that it will be prohibited from sublicensing to a Direct Competitor (to be defined in the Commercialisation Agreement) of the Commercialisation Partner in the Territory without its written consent.
[…]
13. Trademarks and names: Commercialisation Partner will not use the University’s or MPP’s name or logo nor the name of any of the inventors or other principal researchers in any kind of packaging and promotional material other than for the purposes of complying with the Commercialisation Agreement, without the prior written permission of both MPP’s and the University’s authorised representative. Licensed Product manufactured under the Commercialization Agreement will be marked (to the extent not prohibited by law): (i) with a notice that such Licensed Product is sold under a license from the University and MPP; and (ii) with all markings and notices as may be required by applicable law, including in relation to patent and other intellectual property.
